Forschungsschwerpunkte am Institut für Mikrobiologie



Am Institut arbeiten auf dem Universitätscampus Hannover-Herrenhausen drei Abteilungen mit den Schwerpunkten Allgemeine Mikrobiologie / Mikrobielle Biochemie (AG Prof. Thomas Brüser), Zelluläre Mikrobiologie / Mikrobielle Signaltransduktion (AG Prof. Natalia Tschowri) und Ökologische Mikrobiologie / Umweltmikrobiologie (AG Prof. Marcus Horn).
Hinzu kommt noch eine externe Arbeitsgruppe mit dem Schwerpunkt Proteasen und Chaperone bei der Protein-Qualitätskontrolle und Regulation in Bacillus subtilis (AG Prof. Kürsad Turgay) mit Sitz an der Max-Planck-Forschungsstelle für die Wissenschaft der Pathogene in Berlin.
Mit dem Institut eng verbunden ist auch die Arbeitsgruppe Geomikrobiologie / Biohydrometallurgie (AG Prof. Axel Schippers) an der Bundesanstalt für Geowissenschaften und Rohstoffe (BGR) in Hannover.
Allgemeine Mikrobiologie und Mikrobielle Biochemie
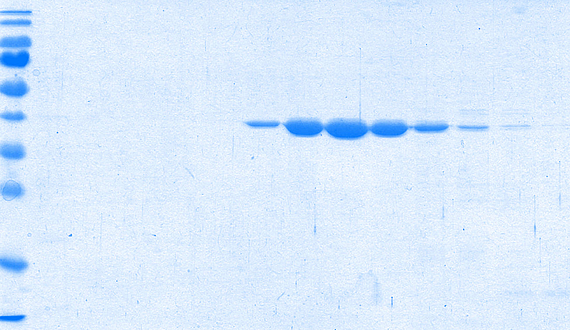
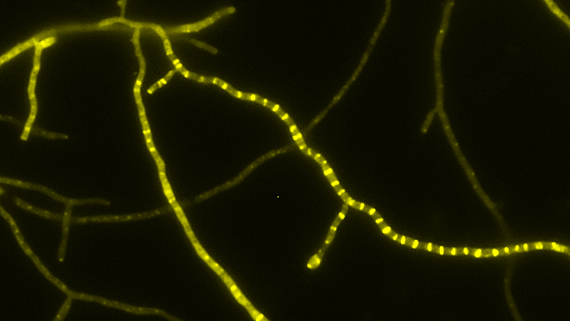
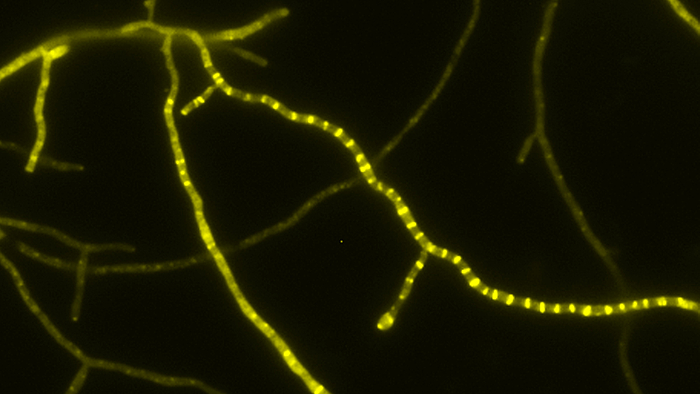
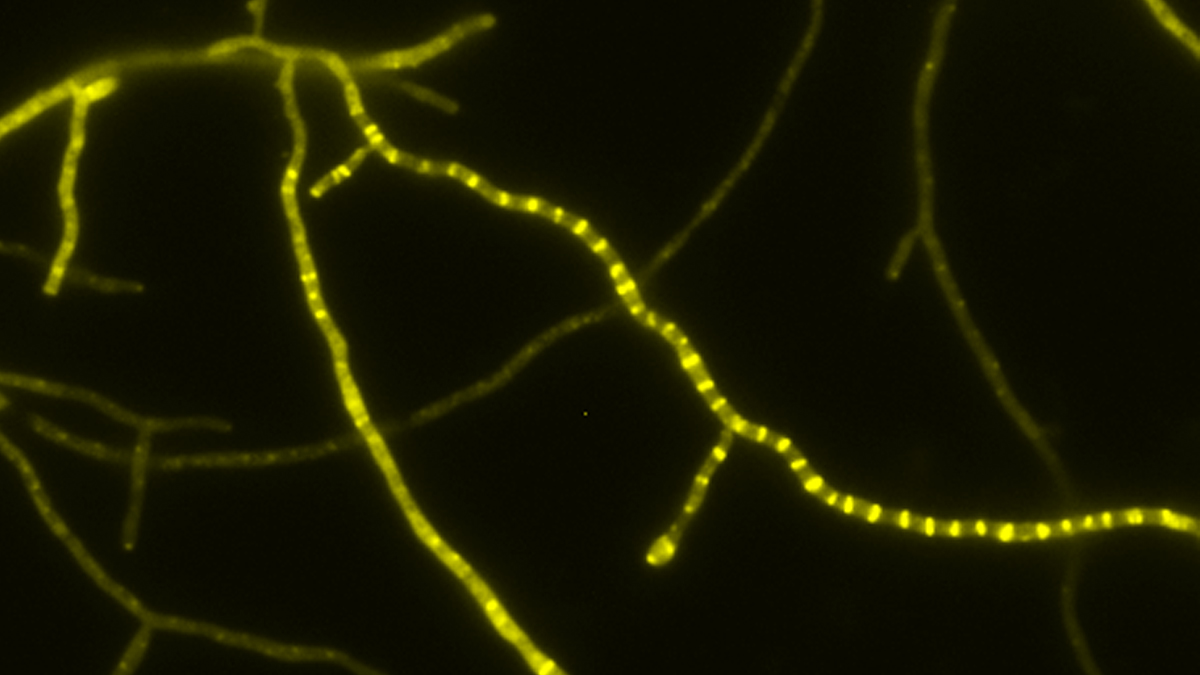
Forschung in der Arbeitsgruppe von Prof. Dr. Brüser:
- Tat transport – Mechanismus des Tat-abhängigen Transports gefalteter Proteine über biologische Membranen in Bakterien, Archaeen und eukaryotischen Organellen bakteriellen Ursprungs
- Pyoverdine – Biochemie der periplasmatischen Maturierungsschritte der fluoreszierenden Siderophore von Pseudomonaden, welche die Eisenversorgung in vielen Habitaten sichern
- Psp system – Wahrnehmung von Membranstress und Signaltransduktion der Psp-Antwort, die zur Stabilisierung der bakteriellen Cytoplasmamembran beiträgt
- Holine – Regulierung und Mechanismus von Phagenholinen und davon abstammenden Transportern, die große gefaltete Proteine ohne Signalpeptide transportieren