Unsere Forschung konzentriert sich auf den Transport gefalteter Proteine über biologische Membranen mit dem twin-arginine translocation (Tat) System bakterieller Cytoplasmamembranen, die Biochemie der periplasmatischen Maturierung von Pyoverdinen in fluoreszierenden Pseudomonaden am Modellorganismus Pseudomonas fluorescens, auf die Wahrnehmung von Membranstress durch Komponenten des Psp-Systems in Escherichia coli und die dadurch generierte Signalkaskade (Psp-Antwort), und auf die Regulation und den Mechanismus der Holin-abhängigen Phagenlyse und den damit verwandten Holin-vermittelten Proteintransport bei Clostridien.
Tat transport
Transport gefalteter Proteine über die Cytoplasmamembran
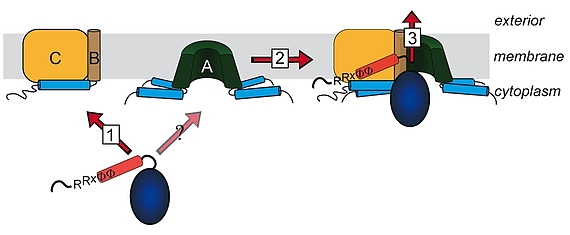
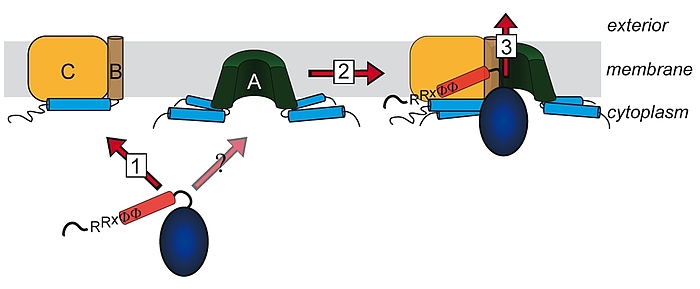
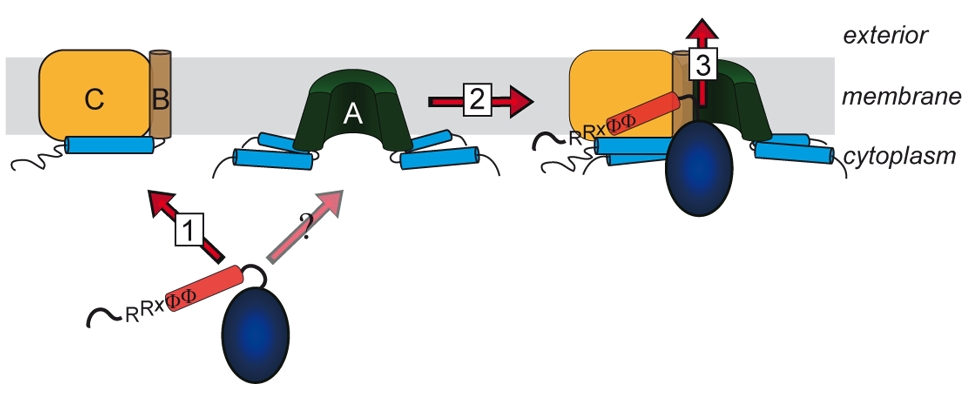
Lange glaubte man, dass nur ungefaltete Proteine über Membranen transportiert werden können. Diese Sicht änderte sich mit der Beobachtung, dass es Proteine gibt, welche als Heterooligomere mit nur einem einzigen Signalpeptid an einer der Untereinheiten transportiert werden können. Die „Co-Translokation“ zeigte, dass die Proteine bereits vor dem Transport gefaltet sein mussten. Die Komponenten des entsprechenden Transportsystems, des Tat-Systems, welches gefaltete Proteine über energetisierte Membranen von Prokaryoten und eukaryotischen Organellen bakteriellen Ursprungs transportiert, wurden identifiziert und TatA, TatB und TatC genannt. TatA und TatB ähneln sich und gehören zur selben Proteinfamilie. Es gibt auch „minimale“ Tat-Systeme aus nur zwei Komponenten, TatA und TatC. Proteine der TatAB-Familie haben eine ungewöhnliche Struktur, mit einem sehr kurzen Membrananker, gefolgt von einer amphipathischen Helix auf der cytoplasmatischen Seite und nur wenigen weiteren Resten mit Bedeutung für den Transport. TatC besitzt sechs Transmembranhelices und bindet fest TatB und weniger fest TatA. Wir arbeiten am Tat-System seit den 1990ern, haben 2003 den „membrane-weakening and pulling“ Mechanismus für den Tat-Transport postuliert und analysieren den Tat-Mechanismus in allen Aspekten.
PSP System
Die Psp-Antwort auf Membranstress
Bakterien unterliegen häufig wechselnden Umweltbedingungen, welche die Integrität der Membranen bedrohen und dann als „Membranstress“ angesehen werden können. Unter vielen dieser Stressbedingungen wird in Escherichia coli ein Regulon hochreguliert, welches aus dem pspABCDE Operon und dem monocistronischen pspG-Gen besteht. Die entsprechenden Promotoren hängen vom alternativen Sigmafaktor Sigma-54 ab, welcher vom bakteriellen „enhancer binding protein“ PspF aktiviert wird, welches divergent oberhalb des pspABCDE Operons codiert ist. PspF wird wiederum von PspA reguliert, dem ersten Genprodukt des pspABCDE Operons. PspA ist der zentrale Regulator welcher auf noch nicht genau bekannte Weise die verschiedenen Stresssignale integriert, um die gewünschte Aktivität von PspF und folglich die gewünschte Regulation der Sigma-54-abhängigen psp-Genexpression zu erzielen. Es wird angenommen, dass die Komponenten PspB und PspC den Membranstress wahrnehmen und mit PspA interagieren, um die Signale weiterzuleiten. Wir interessieren uns für die Signalwahrnehmung durch die verschiedenen Psp-Komponenten, die Signalweiterleitung zu PspA und den Mechanismus der Signalintegration durch PspA. Um diese Aspekte zu analysieren, kombinieren wir ein breites Spektrum struktureller und molekularbiologischer Techniken.
Pyoverdine
Periplasmatische Pyoverdinmaturierung und –modifikation
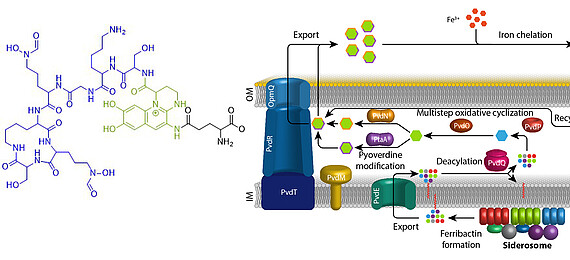
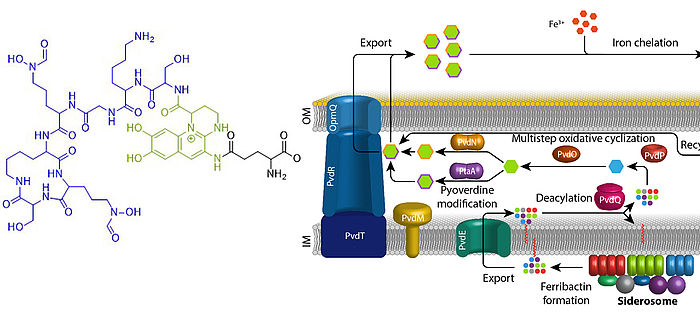
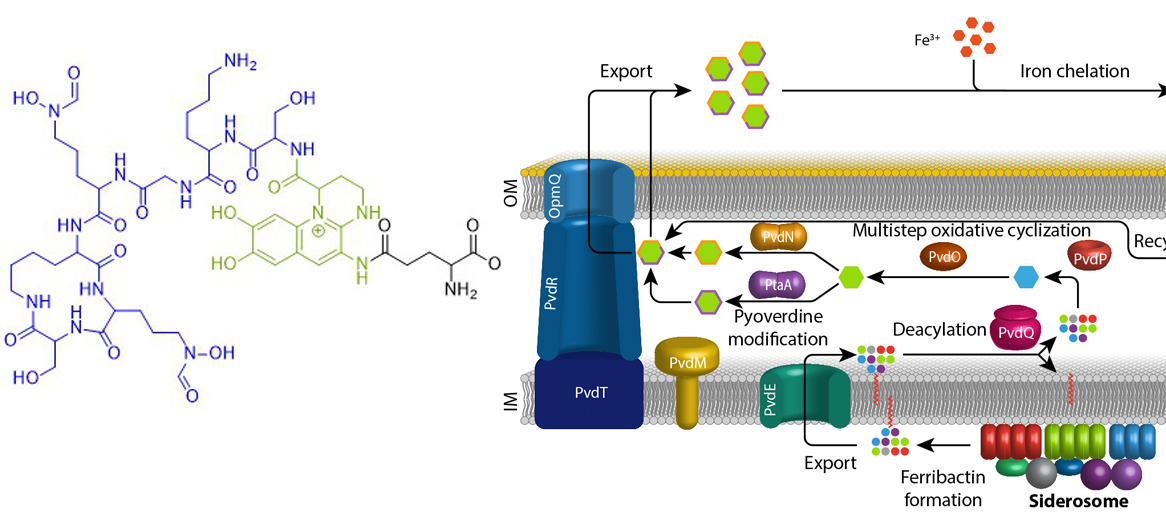
Eisen ist ein essentielles Element für fast alle Organismen auf der Erde. Aufgrund der geringen Löslichkeit von FeIII unter oxischen Bedingungen haben sich spezifische Mechanismen entwickelt, die die Eisenversorgung sichern. Fluoreszierende Pseudomonaden welche in Wirtshabitaten leben, wie etwa humanpathogene oder endophytisch lebende Bakterien, sind extremer Eisenlimitierung ausgesetzt. Unter diesen Bedingungen produzieren und sekretieren diese Pseudomonaden Pyoverdine, fluoreszierende hochaffine Siderophore, welche FeIII komplexieren. Diese Komplexe werden anschließend wieder aufgenommen, um das Eisen in die Zellen zu bringen. Pyoverdine werden aus cytoplasmatisch synthetisierten nicht-ribosomalen Peptiden gebildet, sogenannten Ferribactinen, welche im Periplasma zu fluoreszierenden und weiter modifizierten Pyoverdinen umgewandelt werden. Wir studieren die periplasmatische Maturierung und Modifikation von Pyoverdinen. Bislang konnten wir die physiologischen Funktionen von drei der fünf involvierten Enzyme herausfinden, PvdO, PtaA und PvdN. Wir konnten ebenfalls präziser die Funktion eines vierten Enzymes klären, PvdP, welches gemeinsam mit PvdO die Bildung des Fluorophors katalysiert. Unser Ziel ist die Aufklärung der gesamten Biochemie der periplasmatischen Pyoverdinmaturierung und -modifikation.
Holine
Regulation und Mechanismus von Holinen
Phageninfektionen und Wirtslysen beeinflussen die Population und Diversität von Bakterien in vielen Habitaten. Der Prozess der Wirtslyse wurde über Jahrzehnte studiert und die beteiligten Komponenten sind bekannt. Jedoch sind strukturelle Informationen unzureichend und die Aspekte der Regulation der Wirtslyse sowie der eigentliche Holin-Mechanismus sind weitgehend unverstanden. Als Arbeitsgruppe, die sich intensiv mit der Analyse von Membranproteinkomplexen beschäftigt, haben wir vor einigen Jahren damit angefangen, an der Regulation und am Mechanismus der Holine zu arbeiten, den Phagenproteinen, welche am Ende einer Infektion Endolysine in das Periplasma freisetzen, wo diese die bakterielle Zellwand abbauen und so die Wirtslyse auslösen. Holine können Endolysine entweder direkt in das Periplasma bringen, indem sie entsprechend große Poren bilden, oder sie können die Membran depolarisieren, was Endolysine freisetzt, die nach einem Sec-abhängigen Transport über sogenannte SAR-Domänen zunächst in der Membran verankert wurden. Vertreter der (große) Poren bildenden Holine werden “kanonische” Holine genannt, wohingegen Vertreter der nur die Membran depolarisierenden (kleine) Poren bildenden Holine “Pinholine” genannt werden. Wir arbeiten gegenwärtig an kanonischen Holinen.
Kontakt


30419 Hannover




